Core Technology Experiences
Upcoming Webinar: Join our webinar on Updates on IEC 61368-1:2024 – Product Safety Standards Revision.
Register NowWe Shape Your Ideas into Manufacturing-Feasible Designs with a Focus on Regulatory Compliance
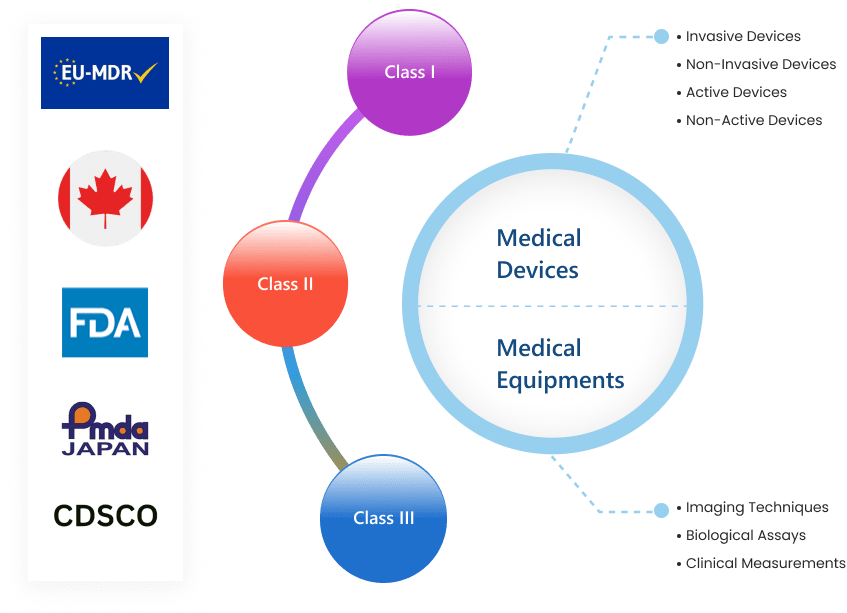
Talk to usOur medical device design services offers concept development, product designing, prototype development, value engineering and obsolescence management to ensure fast-paced market launch and compliance to ISO:13485 guidelines and excellent at meeting regulatory-compliant medical device design processes. Our processes also include maintaining Device Master Files (DMR) to support in regulatory compliance and to easily identify source of error under situations of any non compliance as per FDA. Our medical device team addresses the whole development process from systems, software, electronics, mechanical, quality assurance and regulatory compliance. We can help you optimize your budget and shorten development times of Class l, ll, or lll device using the latest CAD, CAE tools and technologies by our medical device engineers as per FDA, EU and Country specific guidelines.


Our solutions addresses regulatory compliances right from product ideation to marketing in the volatile market
Explore Industries
Leverage our deep expertise in hardware, firmware and software design for instruments.
Visit Page
Our understanding on technologies helps in reducing manufacturing costs, improving robustness in design.
Visit Page
We specialize in considering critical safety aspects during the design of static and mobile equipment.
Visit Page
We prioritize critical usage conditions, applications, and standards in our material handling equipment designs.
Visit Page
We work on Core Disciplines and follow an agile approach and add value in our engagements
Explore Services
We offer small form factor designs to multi-board system designs.
Visit Page
Services for PLCs, HMI, Motion Controllers, Servo Amplifiers, VFD and Plant automation is offered.
Visit Page
Services from compliance management, risk mitigation for medical device manufacturers to meet global market regulations
Visit Page
Virtual reality (VR), augmented reality (AR) and Mixed reality connect the virtual and physical worlds. They allow you to visually absorb information
Explore Services
Reach us for a comprehensive product/process documentation to different stakeholders.
Visit Page
We engage with Global Captive Centre’s by addressing Talent Crunch in Electronics and Mechanical Engineering domains.
Visit PageThe "News & Updates" section features the latest developments and innovations in our services and solutions, including engineering services, product compliance, environmental regulations, Obsolescence Management, and regulatory & product safety.

In today's competitive market, businesses need every advantage to stand out and connect with their customers
View Blog
The Carbon Border Adjustment Mechanism (CBAM) is a transformative EU policy designed to curb global emissions
View Whitepapers
Join us for an exclusive regulatory update webinar on the latest revision of IEC 61368-1:2024, the international standard governing product safety requirements for electrical and electronic equipment.
View Webinar
Explore the pervasive presence of PFAS, their impact on our homes and environment, health risks, and the regulations in place to control them.
View Videos
A prominent provider of engineered carbon dioxide (CO₂) storage and distribution systems
View Case StudiesSubscribe to our Newsletter and Product & Service Updates.
By submitting, I agree to receive newsletters and updates from iLenSys Technologies and can unsubscribe at any time. View Privacy Policy.
Existing Preferences
Interested Topics:
Subscription Preferences:
Latest Preferences
Interested Topics:
Subscription Preferences:
Please review your preferences. The Existing Preferences reflect your current selections. The Latest Preferences show the new choices you've made.
If you're happy with the new preferences and ready to update them, click Confirm. If you wish to review or alter your choices before finalizing, click Close and adjust as necessary.
We'd love to hear from you

Our websites require some cookies to function properly (required). In addition, other cookies may be used with your consent to analyze site usage, improve the user experience and for advertising.
Welcome to iLenSys Technologies Pvt. Ltd.'s Cookie Settings. We respect your privacy and want to give you control over your online experience. Please select your cookie preferences below:
These cookies are necessary for our website to function and cannot be turned off.
Help us improve our website by allowing us to gather anonymous usage data. Turning off these cookies won't affect your experience.
These cookies remember your choices on our site for a more personalized experience. You can turn them off if you prefer.
We use these cookies to show you relevant ads. Turning them off won't stop all ads, but they may be less relevant to you.
Your choices will be stored as cookies on your device. If you clear your cookies, your preferences will be reset.
iLenSys Technologies Pvt. Ltd.
8-2-293/82/L/231/ABC,
MLA Colony, Road No: 12, Banjara Hills,
Hyderabad, Telangana 500 034
Phone: 040 – 66998246, 040 – 66998234
Email: info@ilensys.com



